Dr. Aikeremu Ahemaiti
Department of Neuroscience, Uppsala University, Sweden
Background
Dr Ahemaiti is a researcher in the Department of Neuroscience at Uppsala University, working for multiple research groups. Dr. Ahemaiti works with Prof. Malin Langerström researching mouse spinal cord sensory circuits responsible for sensations such as pain and itch, and with Prof. Henrik Boije researching zebrafish motor function and regulation, again focusing on the spinal cord. Both studies concern neural networks and elucidating function using cell biology and electrophysiology, as well as genetic sequencing on single cells.
These studies use both electrophysiological patch techniques along with fluorescent imaging of cells and networks.
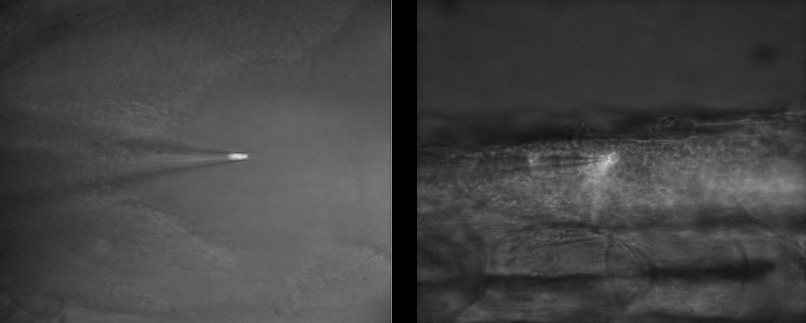
Challenge
In electrophysiology studies involving patch pipettes, it is important to balance the fluorescence imaging with brightfield, as the latter is needed to direct the pipette towards the cell of interest. This means that fluorescent signals can often be lost in the more intense brightfield illumination, meaning a highly sensitive camera is needed to pick out the fluorescent signal.
A previous CMOS solution was also presenting with patterns and artifacts, which interfered with the cell imaging. A back-illuminated CMOS camera with a single sensor and a clean bias would also be necessary to remove artifacts and improve the signal-to-noise ratio.
Imaging both mouse and zebrafish models with different optical and electrophysiological experiments also lend to the challenge, meaning a highly flexible camera solution is needed, especially one with a larger FOV for future experiments. The different fluorescent dyes used are also different between models, with mouse experiments using bright tdTomato probes to label the cytosol, and zebrafish using less intense GFP and mCherry that are more localized to membranes. All this variability needs to be matched with an imaging solution.
With previous cameras I was using 50 ms exposures, now with the [Prime BSI Express] I am using 2 ms so there is a big difference there.
Solution
The Prime BSI Express is a highly flexible camera for electrophysiology studies, with high speed, near-perfect 95% quantum efficiency, and low read noise. The balanced pixel size also allows for the Prime BSI Express to thrive in a range of different experiments, with a large FOV and high resolution to match the high sensitivity.
The high dynamic range 16-bit mode allows for imaging of both brightfield and fluorescence, allowing for Dr Ahemaiti to see both tagged cells and the electrophysiological patch pipette at the same time, even if fluorescent signals were low. The Prime family of cameras comes with high image quality, meaning a single sensor with no split, no patterns, and no artifacts, resulting in a high signal-to-noise ratio for sensitive optical and electrophysiological experiments.
Dr Ahemaiti also commented on the simplicity of the Prime BSI Express, “Setup was simple thanks to online instruction and previous remote demo sessions… it was easy to turn on and use thanks to USB connection.”

