Dr. Ji-Gwang Hwang
Department of Accelerator Physics, Helmholtz-Zentrum Berlin
Background
Dr. Hwang is participating in the Athena_e project, a collaboration between Helmholtz Centers for the technical evolution of plasma wake-field accelerators in conjunction with normal-conducting accelerating structures. Helmholtz-Zentrum Berlin (HZB) operates an advanced synchrotron light source, BESSY II, as well as the low energy storage ring Metrology Light Source (MLS). HZB and Dr. Hwang have a role in the development of high-sensitivity beam profile monitors with a micrometer spatial resolution.

Challenge
Achieving a spatial resolution of a few micrometers is only feasible by performing profile detection with interferometric techniques (slits and wavelength filters), but these techniques can reduce light intensity produced by the synchrotron by two orders of magnitude, making detection a challenge. Any detector needs to be carefully evaluated, especially for quantum efficiency (QE), read noise, and any nonlinear behavior.
The influence of QE and read noise on the determination of beam size as a function of the number of photons was estimated quantitatively by a Monte-Carlo based numerical simulation. The results showed that a high QE significantly improved the resolution compared to the read noise at low-intensity levels. In addition to this, the background noise compensation is important since the photons per pixel are from 4 to 20 which has a nonlinear response due to statistical noise. Thus a new state-of-the-art camera that has a back-illuminated sensor and low readout noise is highly demanded.
A new state-of-the-art sCMOS, the Prime BSI camera with back-illuminated sensor and low readout noise was highly demanded.
Solution
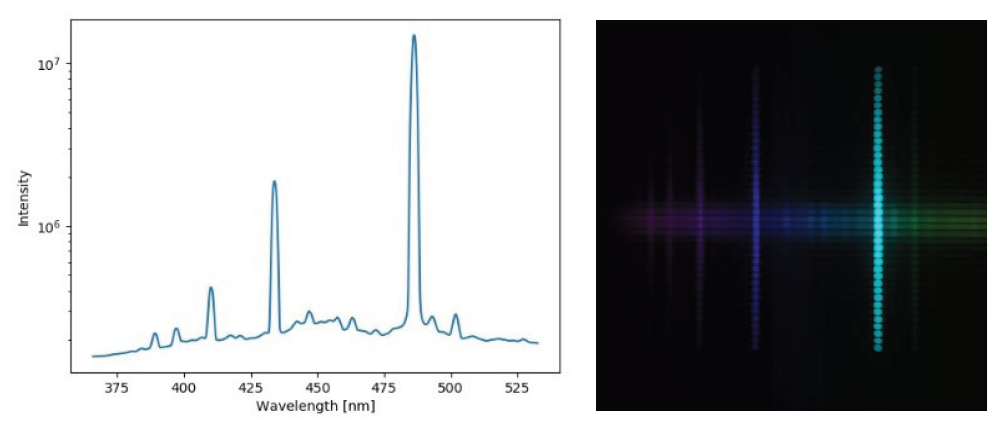
Dr. Hwang chose the Prime BSI sCMOS for their detector needs, testing the camera on an infrared beamline at MLS.
Various filtering techniques including the BSI’s internally available despeckle function, were applied to filter speckles and singular noise. As mentioned by Dr. Hwang: “The Prime BSI is capable of retrieving interferometry fringes with a peak intensity of ~3 photons/pixel. The measurement limit owing to the read noise and QE of the detector is quite consistent with our estimation predicted by the numerical simulation.”