Professor Johannes Huppa
Iago Doel-Perez, MSc
Institute of Hygiene and Applied Immunology, Medizinische Universitat Wien, Austria
Background
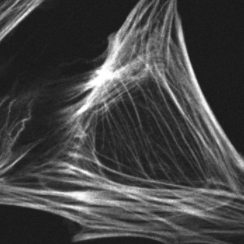
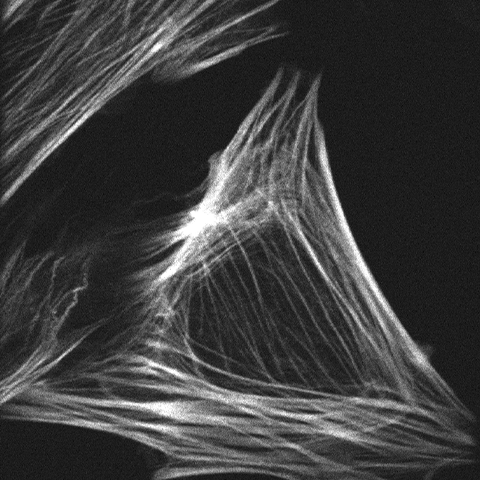
Prof. Huppa and team study T cells, cells that play an important role in the immune response. These T cells can recognize antigens and distinguish between friend (normal cells) and foe (viruses or infected/dangerous cells) in our bodies. By studying this recognition of antigens by T cells, Prof. Huppa and the team aim to discover more about the innate and adaptive immune system.
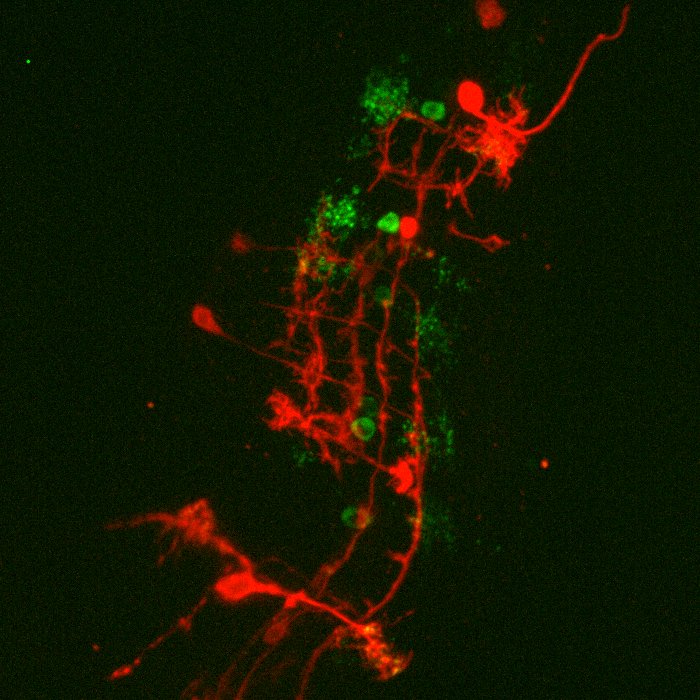
Ph.D. student Iago Doel-Perez outlined his research: “We work with both human and mouse T cells, studying the interaction of live T cells with a platform that allows us to control the activation of T cells.” When these T cells activate there is an influx of calcium ions (Ca2+), meaning that calcium imaging presents an easy and quick discrete marker to show activation and deactivation of T cells through fluorescent imaging.

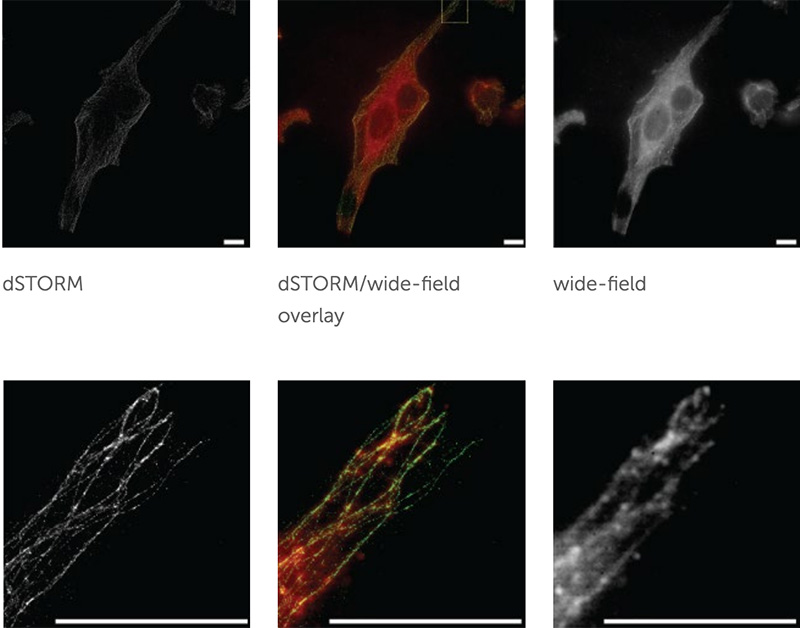
Left) T cell activation. Blue T cells are inactivated (base calcium) and green T cells are activated (increased calcium as shown by the scale in AU).
Right) Same cells but with a localization tracker, showing the direction and distance the T cells moved, as well as the velocity (color corresponds to the scale below the image, units are μm per second).
Challenge
Iago explained: “We want to record the moment that the T cells activate in a controlled presentation manner, so we know what we are providing the cell with as information and then we can check the output of this cell. How fast does the activation happen and how many cells?”
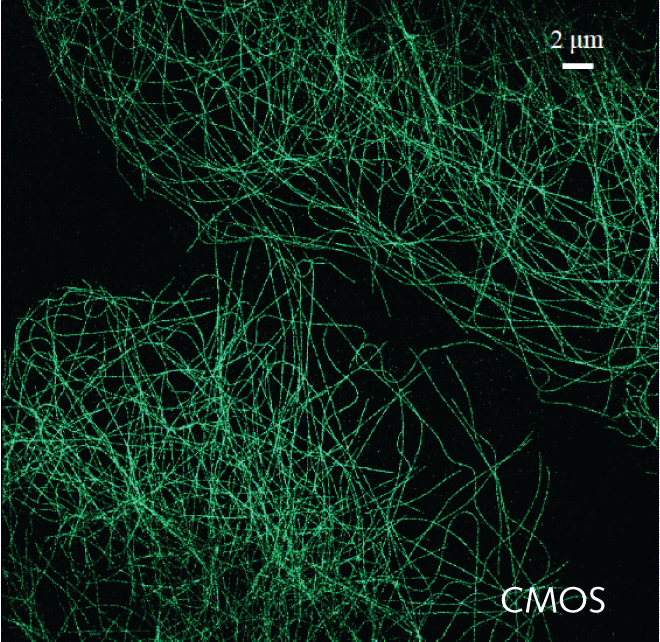
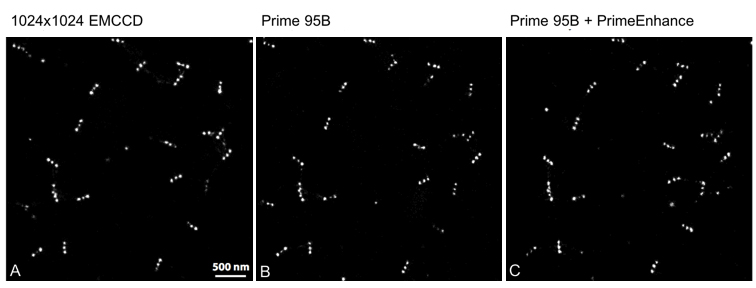
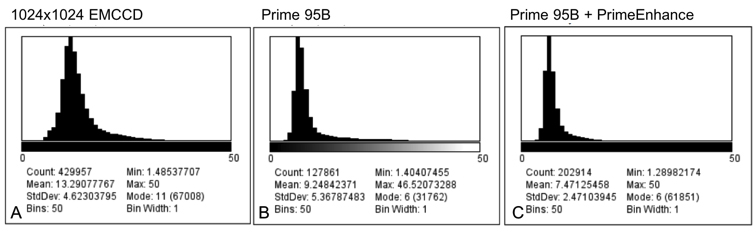
Iago uses the calcium indicator Fura-2 to determine when the T cells activate and in order to analyze the number of active cells and their behavior. For this, Prof. Huppa and the team need a camera with a wide field of view in order to image as many cells as possible. Iago recalled: “With a previous EMCCD camera we could only record a quarter of the area so we imaged four times less cells so we could not test as many conditions.”
With our previous EMCCD we could only record a quarter of the area… the [Prime 95B] allows us to record many cells at a higher resolution
Solution
Iago told us his experience with the Prime 95B: “A big advantage is the larger field of view and also the smaller pixel size than our EMCCD, we can really capture cells on a very wide area, which allows us to record many cells and get an appropriate statistical description of the system… The large field of view combined with the higher resolution let us use a very low magnification, this is a really big advantage with the [Prime 95B] camera.”
Iago went on to mention: “The camera was very easy to work with, we are using μManager and everything worked out of the box.”