Dr. Alexander Carr
Dr. Steven Lee, Principal Investigator
The Lee Lab, Department of Chemistry, University of Cambridge
Background
TheLeeLab, at the University of Cambridge, focuses its research on developing biophysical tools to answer fundamental biological questions, primarily using single-molecule fluorescence imaging techniques. Recently the group has established single-molecule spectroscopic imaging, facilitating local hydrophobicity mapping (1), as well as implemented cutting-edge point-spread function engineering for large-volume single-particle tracking in live T cells (2).
Challenge
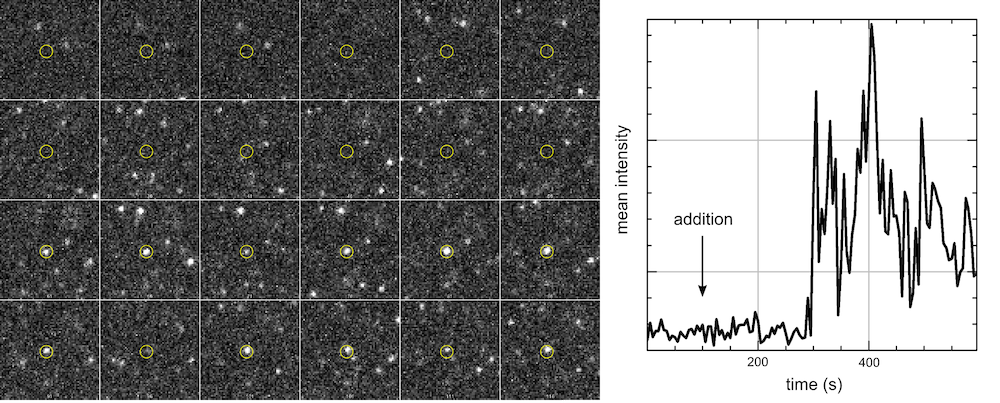
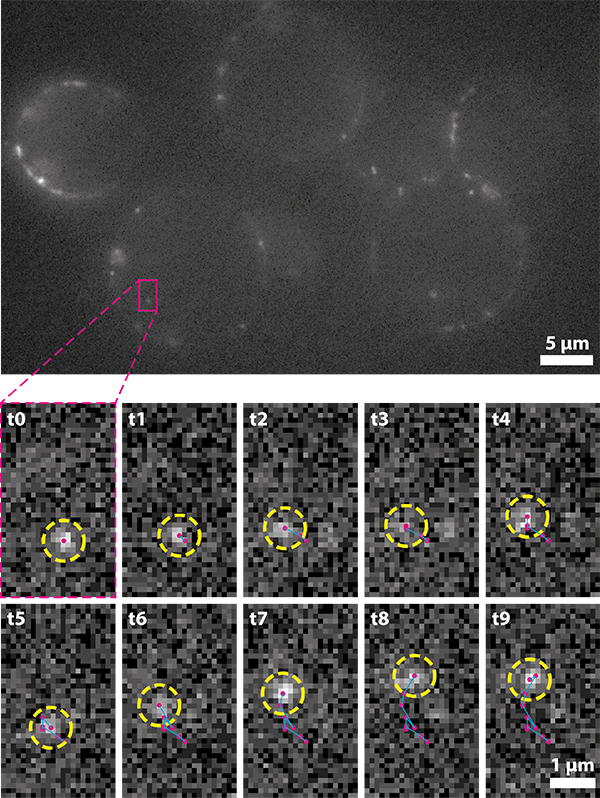
As with all single-molecule techniques, sensitivity is vital as the photon budget of individual fluorophores can be limited. For single-particle tracking applications, detecting more photons allows for longer trajectories to be recorded and more robust statistical analysis to be conducted.
Additionally, the motion of a fluorophore must be adequately sampled in order to accurately record its behaviour. For fast-moving targets, such as many cytoplasmic and nucleic proteins, short exposure times are required and thus the emission is spread over a greater number of frames.
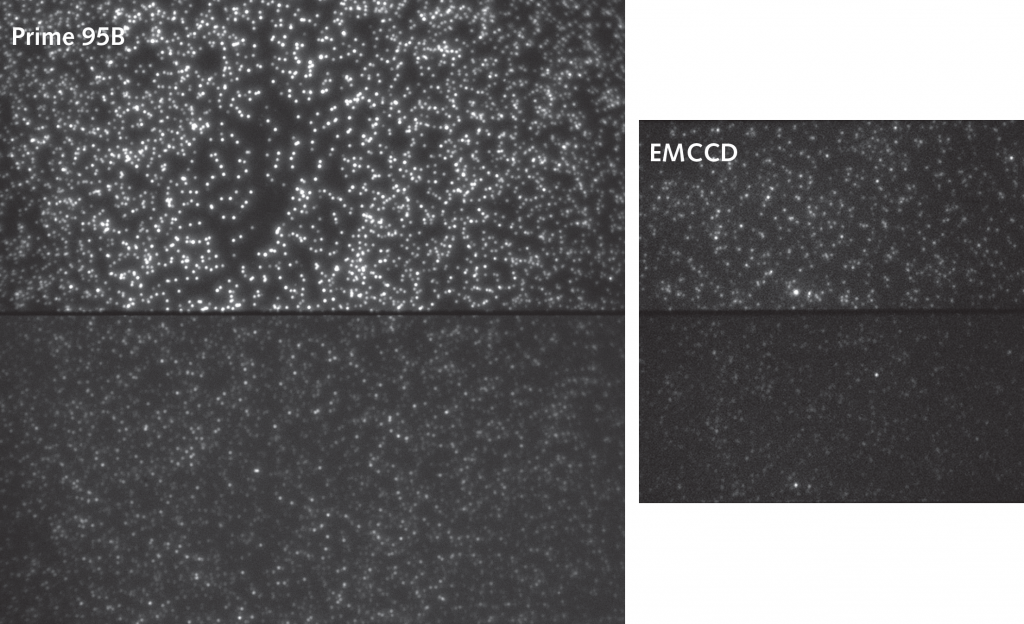
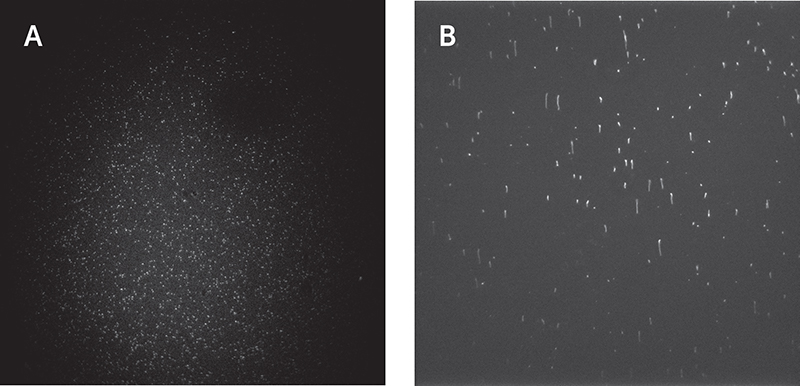
In order to achieve live single-particle tracking of fast-moving targets, both high sensitivity and fast acquisition rates are vital. Although EMCCDs have previously been used to achieve both of these factors, high speed came at the cost of a much reduced field of view, which made data collection inefficient.
The Prime BSI Back-illuminated sCMOS gives EMCCD-level sensitivity at high frame rates and with a very large field of view, speeding up our single molecule tracking acquisition.
Solution
The high quantum efficiency and low noise of the Prime BSI sCMOS combines EMCCD-level sensitivity with a fast acquisition rate and much larger field of view. The Prime BSI speeds up data collection as multiple cells can be imaged within the same field of view at >100 fps with high contrast.
- Bongiovanni, M.N., J. Godet, M.H. Horrocks, L. Tosatto, A.R. Carr, D.C. Wirthensohn, R.T. Ranasinghe, J.-E. Lee, A. Ponjavic, J. V. Fritz, C.M. Dobson, D. Klenerman, and S.F. Lee. 2016. Multi-dimensional super-resolution imaging enables surface hydrophobicity mapping. Nat. Commun. 7: 13544.
- Carr, A.R., A. Ponjavic, S. Basu, J. McColl, A.M. Santos, S. Davis, E.D. Laue, D. Klenerman, and S.F. Lee. 2017. Three-Dimensional Super-Resolution in Eukaryotic Cells Using the Double-Helix Point Spread Function. Biophys. J. 112: 1444–1454.