Dr. Johannes Hohlbein
Laboratory of Biophysics, Department of Agrotechnology and Food Sciences
Wageningen, The Netherlands
Background
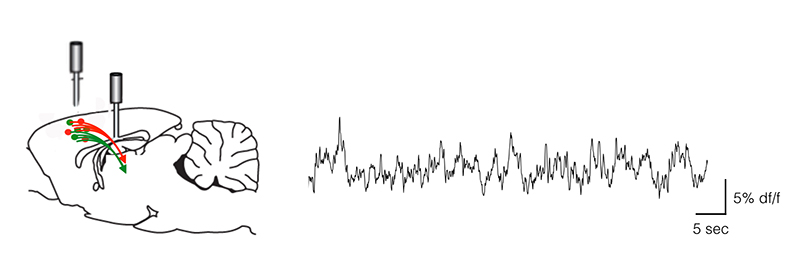
The Hohlbein Lab uses techniques such as single molecule imaging, FRET and super-resolution PALM/STORM to investigate a variety of topics. These include increasing the throughput of single-molecule measurements by using fluidic devices in combination with TIRF microscopy, using single-molecule FRET to study binding DNA-protein interactions and probing anisotropic food structures using single particle diffusometry.
Challenge
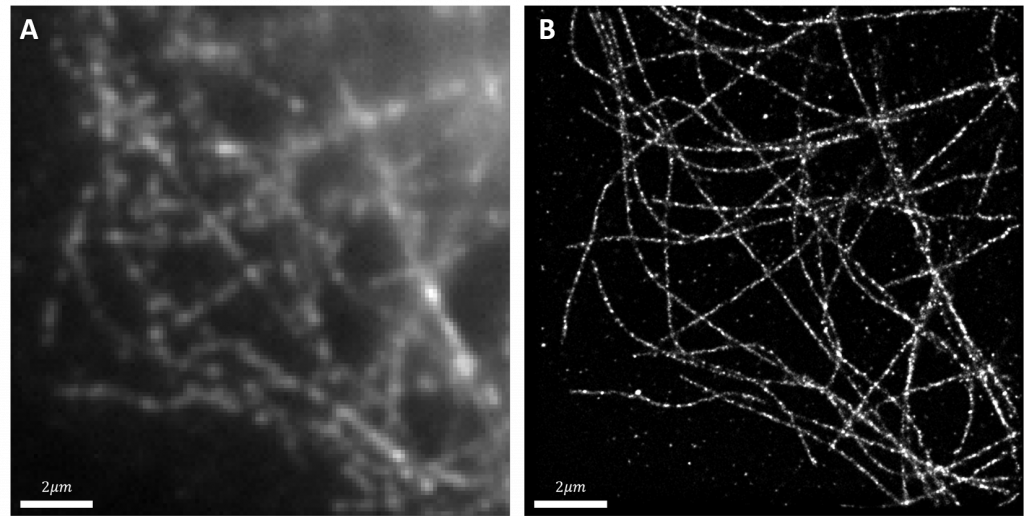
The Hohlbein Lab typically struggles with the limited number of emitted photons in their single-molecule imaging and smFRET experiments, therefore a camera with a high quantum efficiency is advantageous for these applications.
In addition to this, Dr. Hohlbein would like to image the largest field of view as possible with the system when using a 100x objective to achieve the maximum throughput in data acquisition.
The 95% quantum efficiency of the back-illuminated sensor ensures that almost all photons arriving at the camera are collected which is of great benefit for our lowest light applications
Solution
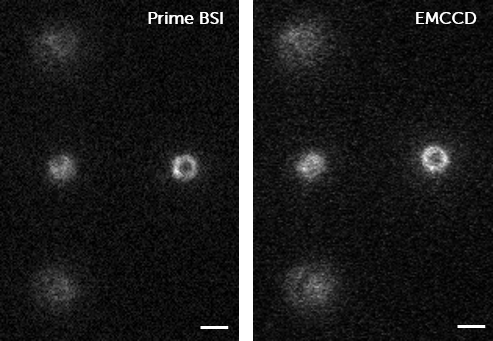
Dr. Hohlbein chose the Teledyne Photometrics Prime 95B back-illuminated sCMOS for a number of reasons. He told us, “Firstly, the 95% quantum efficiency of the back-illuminated sensor ensures that almost all photons arriving at the camera are collected which is of great benefit for our lowest light applications.”
He went on to say that, “The pixel size of 11 × 11 µm ensures that with the 100× magnification of the optical system, we have an ideal 110×110 nm mapped from the sample plane onto each camera pixel, fulfilling the Nyquist sampling criterion and maximizing the field of view. With the camera sensor size of the Prime 95B which consists of 1200×1200 pixels, our usable area is around 5 times higher than with a conventional 512×512 pixel EMCCD camera.”
The lab is planning to make use of PrimeLocate™ in the future, which automatically detects the brightest pixels in the image and only transfers this data to the computer. Dr. Hohlbein told us, “With PrimeLocate, we will have an intriguing option to reduce the storage requirement of our raw data as ROIs are automatically detected and selected for saving.”