Prof. Richard Börner, Mr. Anxiong Yang
Laserinstitut, Hochschule Mittweida University, Germany
Background
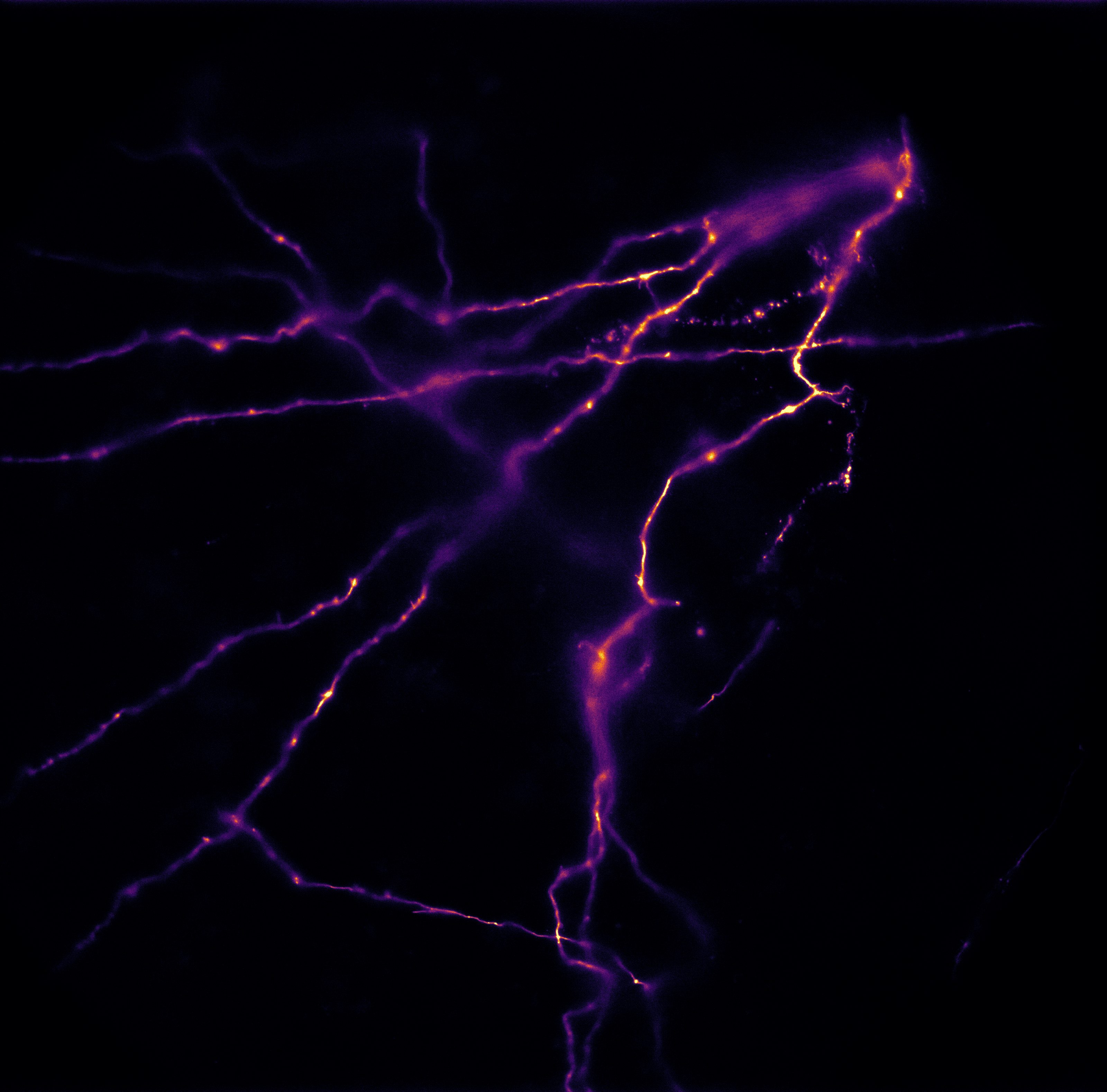
The lab of Prof. Borner is involved with studying the dynamics of RNA molecules using a range of single-molecule imaging techniques, including single-molecule Forster resonance energy transfer (smFRET) and total internal reflection fluorescence (TIRF) microscopy.
Anxiong Yang from Prof Borner’s group has built a custom two-color smFRET imaging system in a TIRF microscope that uses stroboscopic alternating laser excitation (sALEX) to monitor thousands of RNA molecules in parallel, with time resolutions down to 1 ms.
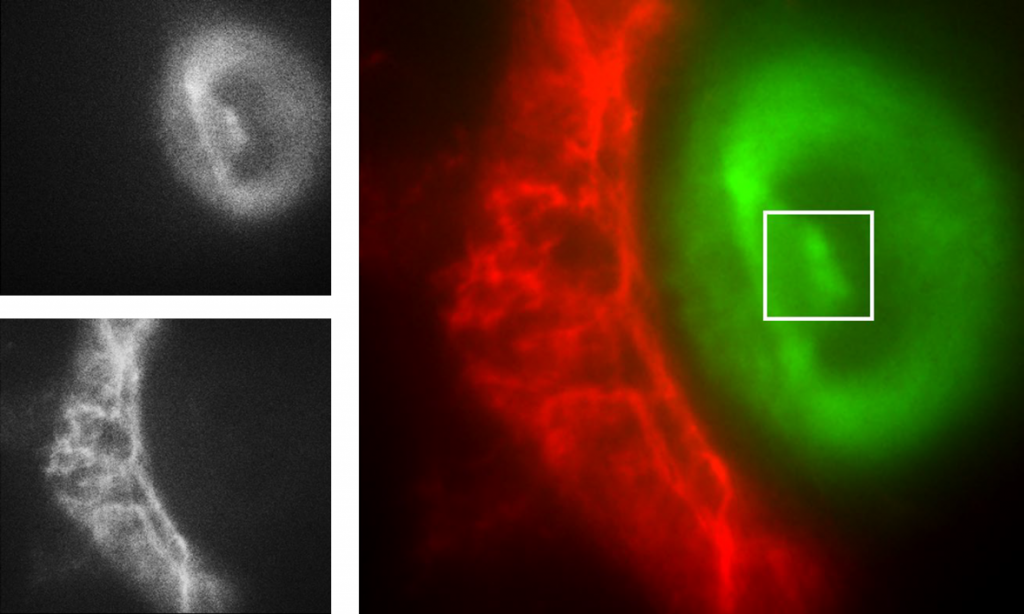
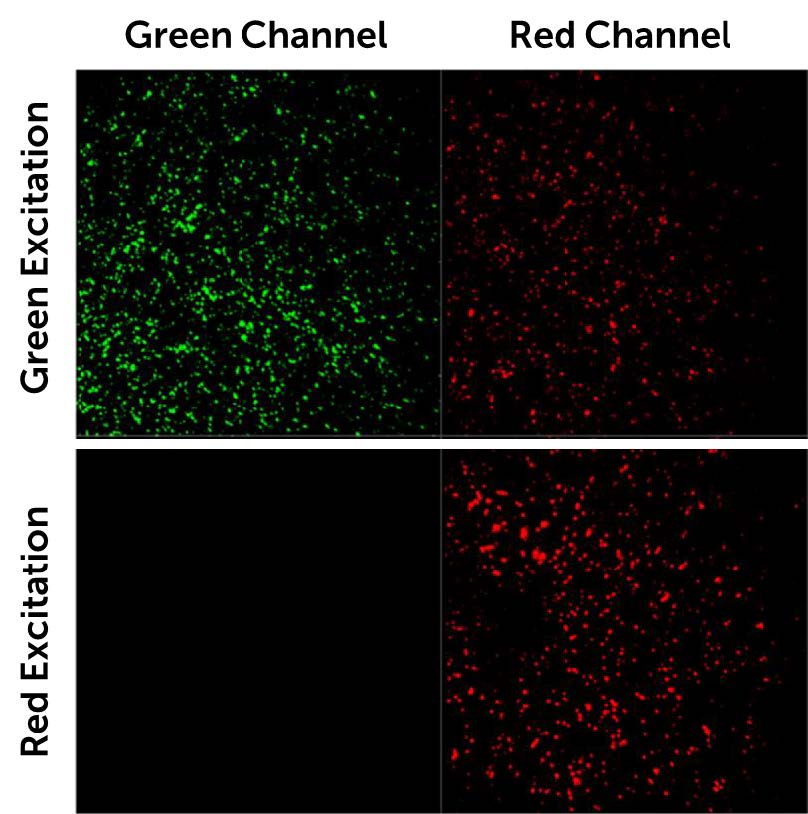
Mr. Yang further explained his imaging work, “We immobilise molecules onto a coverslip with two dyes, Cy3/5, fluorescence-labelled molecules. Then we use a microfluidic system to adjust the buffer conditions (salt concentration, pH, metabolite concentration) within the sample chamber, and image these surfaces with our TIRF microscope to observe dynamic and optimise or automate the immobilisation process.”
Challenge
Mr. Yang told us about the challenges of these experiments, “We want to capture a big area of our sample to get as much data as we can, because sample preparation is complex and very time-consuming. We want to get more information from our samples.”
“We split the fluorescence emission light in two channels, green and red, and want to image them using each half of the camera sensor. Also, we are using an alternating laser excitation (ALEX) box and we use the TTL output trigger signal of the Prime BSI Express sCMOS to digitally modulate our two laser sources.”
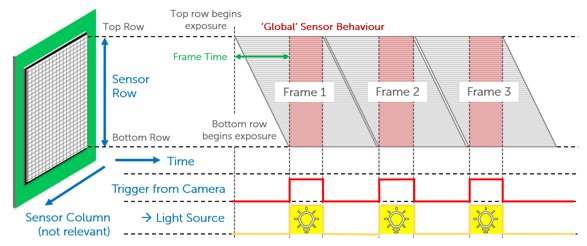
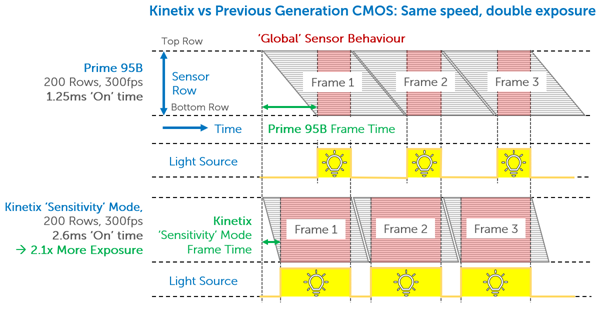
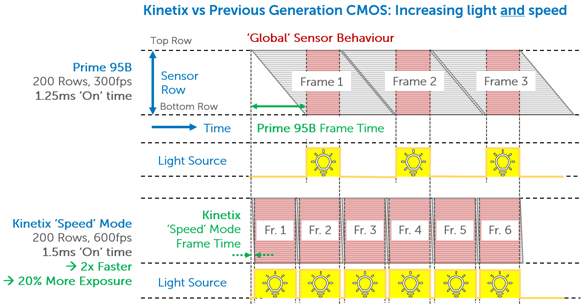
If splitting the sensor to simultaneously image two channels, a camera sensor will be halved in size, meaning a large FOV is paramount to maximize data capture even when reduced in size. Alongside this, this research requires advanced external hardware triggering capabilities in order to control two light sources with alternating excitation.
The [Prime BSI Express] sensor captures a really large area of our sample, and the noise levels are very low, these are important for us.
Solution
The Prime BSI Express CMOS camera is an ideal solution for this application, with high sensitivity thanks to a combination of low-noise CMS mode and 95% quantum efficiency for signal collection. The Prime BSI Express acquires at high speed even in CMS mode. and has a sufficiently large sensor to work well with a splitter and capture two channels simultaneously with thousands of molecule events across each.
Mr. Yang described his experience with the Prime BSI Express, “Your camera sensor is big enough to capture a really big area from our sample, the background signal and noise levels are very low; we can also control the temperature using the fan, this is important for our experiments with fluorescent light detection.”
“We are using Micro-Manager open-source software to run the whole system. and it works great with your camera and our lasers. I can set everything up in the software. The camera triggers also work great with our lasers and system. We have also 3D printed a camera holder for the Prime BSI Express] to match the height of our detection beam pass. as we made the system with this camera in mind.”