Dr. Tim Schröder, Mr. Maarten van der Hoeven
Integrated Quantum Photonics Lab, Humboldt-University of Berlin, Germany
Background
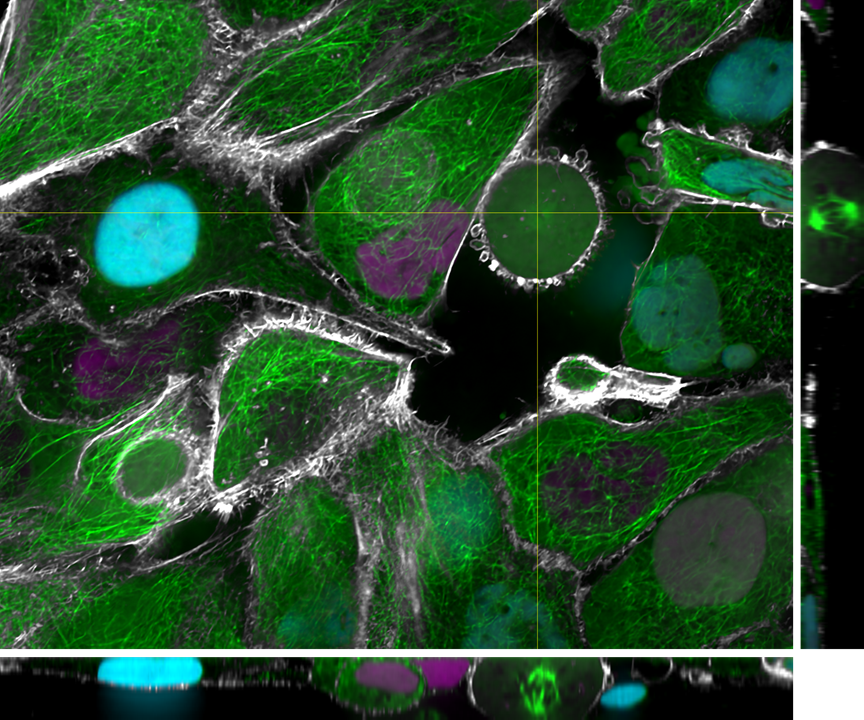
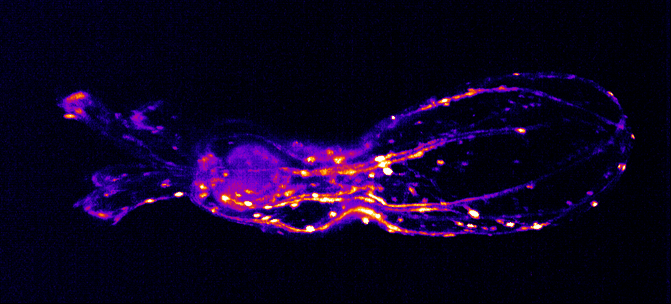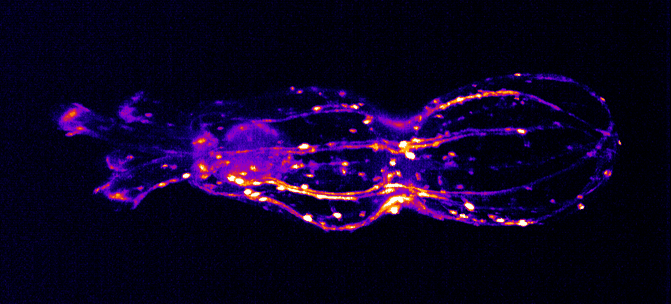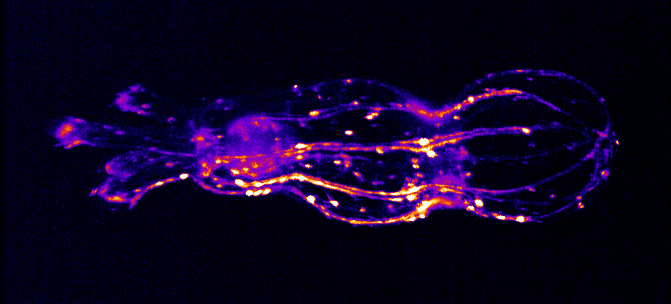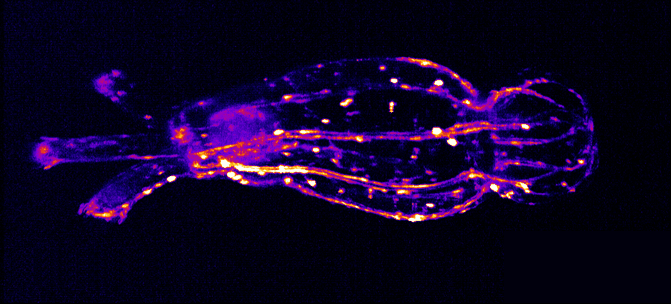
The Integrated Quantum Photonics lab of Dr. Tim Schröder at the Humboldt-University of Berlin is interested in understanding, controlling, and developing use cases for quantum research. In this particular project, Maarten van der Hoeven is characterizing and studying the behavior of color centers in diamond nanostructures. These color centers are extremely stable single photon sources that can be utilized to build quantum sensors or quantum communication devices with high communication rates. To achieve this, Maarten searches for ways to couple those quantum systems to collect transmitted photons as effectively as possible.
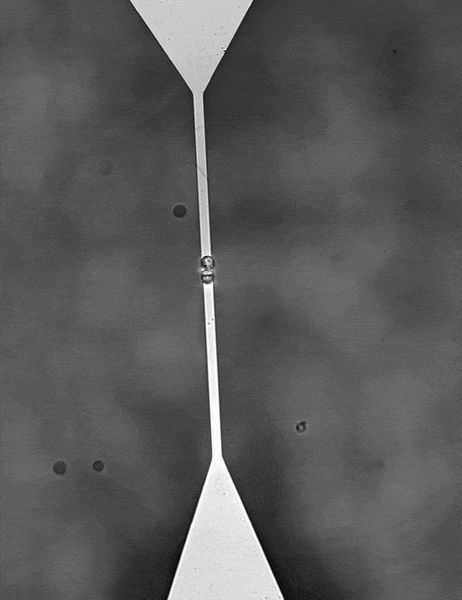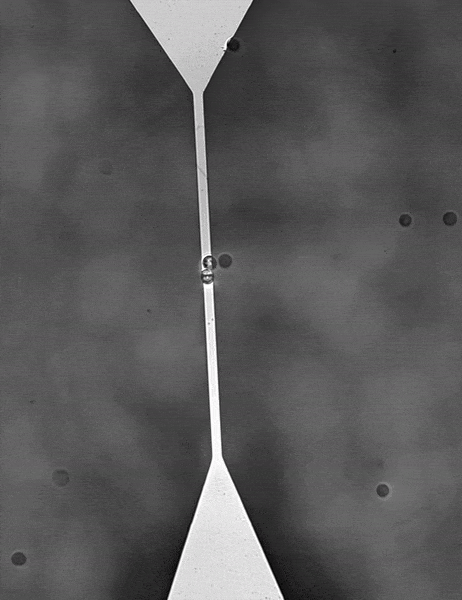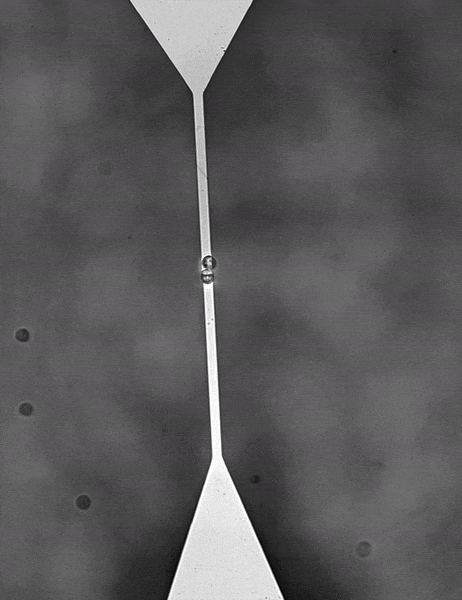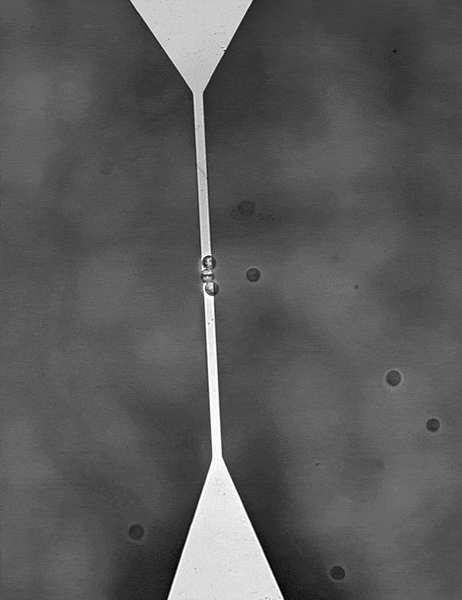
For color centers in diamond, the fabrication of nanostructures that contain emitters is a well-known method for enhancing the extraction of photons. These nanostructures can be used for single-mode fiber coupling of the color center’s emission, as it is a requirement for high photon collection efficiency and a necessity for integrated systems.
Challenge
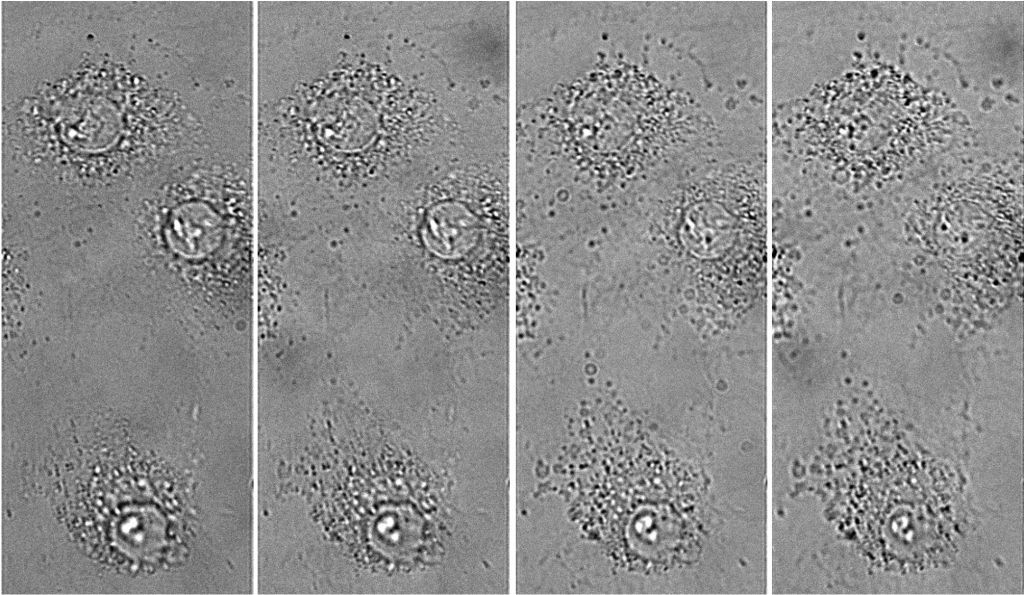
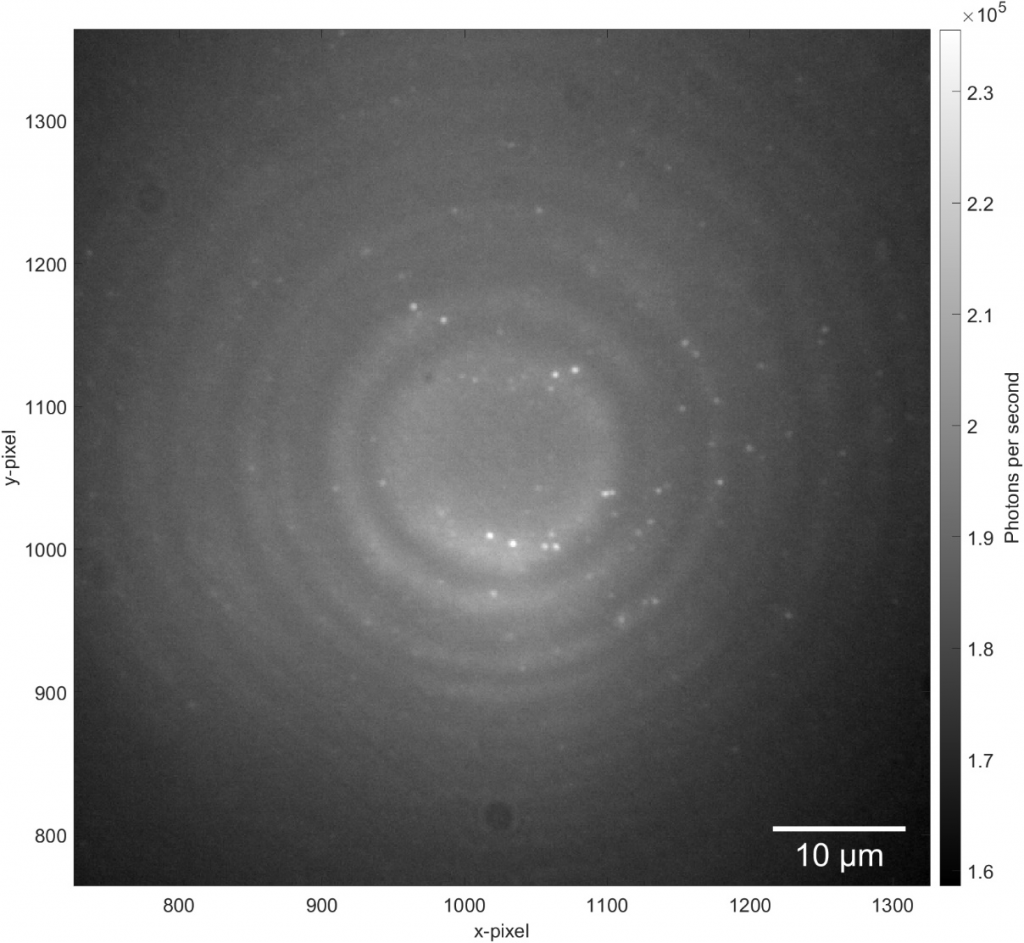
In order to detect individual point sources, which are low in signal down to a few photons per millisecond, a camera with a very high quantum efficiency and very low read noise is required. A confocal raster scanning method was previously used, which was much slower than simultaneously locating many tens or hundreds of color centers.
A well-controlled scientific camera sensor gives reliable access to a quantifiable number of detected photons. Other, less optimized camera solutions do not easily allow for quantitative analysis but require frequent calibration and/or contain patterned artifacts in offset and image leading to worse results. Moreover, for some experiments, it is crucial to have a large enough full well capacity to obtain recordings of various signal levels from very dim to very bright – only possible with a high bit-depth and well capacity.
Lastly, the color centers are at random locations and require locating in respect to landmarks on the diamond so further processing can be performed. A large field of view sensor would be truly beneficial as it speeds up the entire process and makes it very repeatable.
With the [Prime BSI] on our custom setup we can carry out experiments which we could not perform before.
Solution
Maarten told us that the Prime BSI sCMOS camera is a very good solution for their imaging needs, as individual color centers can be reliably identified, reproducibly located and characterized. The Prime BSI has become an established solution of choice on the color center setup, and this group has recently purchased a second camera for a new system.
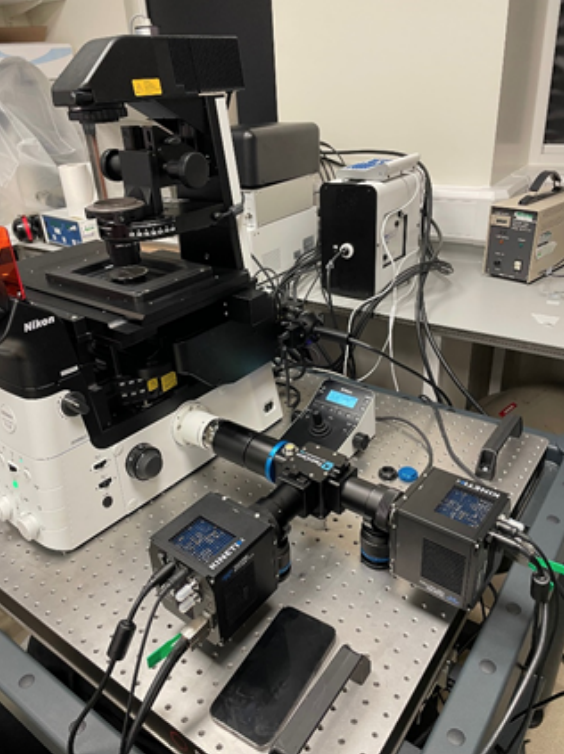
As well as using a Teledyne Photometrics Prime BSI sCMOS camera for widefield acquisition, this self-built setup also includes a Teledyne Princeton Instruments SpectraPro HRS 500 spectrometer with a ProEM EMCCD. As spectroscopy data can only be revealed at the locations where events are situated, a very precise localization needs to take place based on the camera images and relayed with a high precision XY-stage in order for the spectroscope to measure at the intended positions requiring a delicate interaction between all components controlled by a single software interface. The fast and accurate combination of the Prime BSI and SpectraPro enables reliable identification of the nature of individual color centers. With its astigmatism-corrected light path and the flexibility to switch and interchange grating turrets, the SpectraPro HRS is the ideal partner for the Prime BSI on this system.
Overall, Maarten said that “[we] can carry out experiments with our self-built setup which we could not perform before. Further efforts will be made to improve the entire system even more in order to gain additional insight into color centers, their behavior and eventually lead to technological progress in our understanding and use of them in the scope of the groups research focus.”