Roman Barth
Cees Dekker Lab, Department of Bionanoscience, Delft University of Technology, Netherlands
Background
The Cees Dekker lab at TU Delft works with single-molecule imaging techniques in order to explore life at the nanoscale. Roman Barth is a PhD candidate in the Cees Dekker Lab, running experiments using the two TIRF single-molecule imaging systems in the lab.
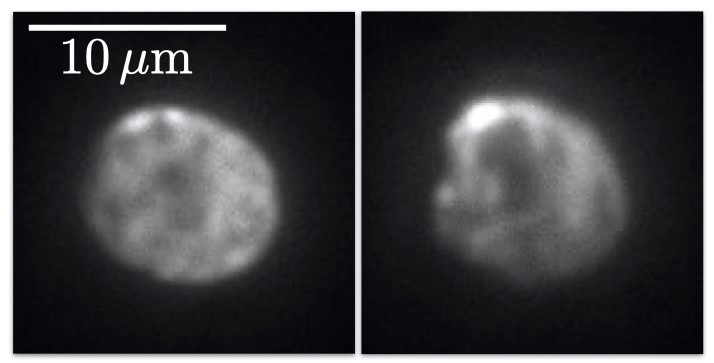
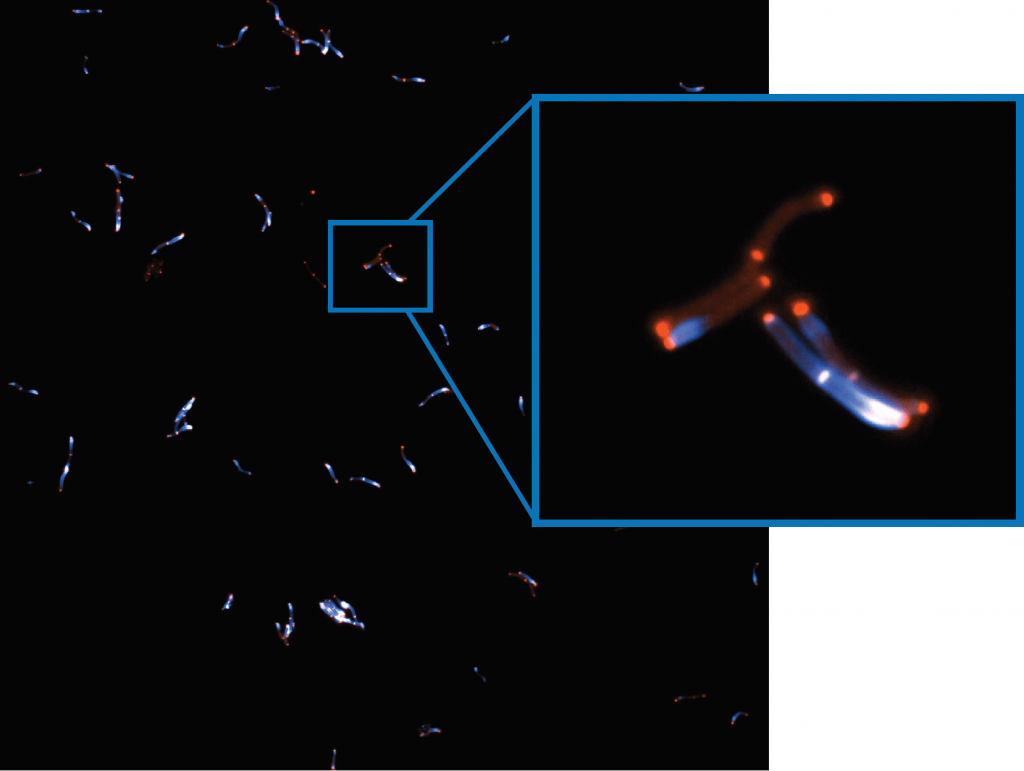
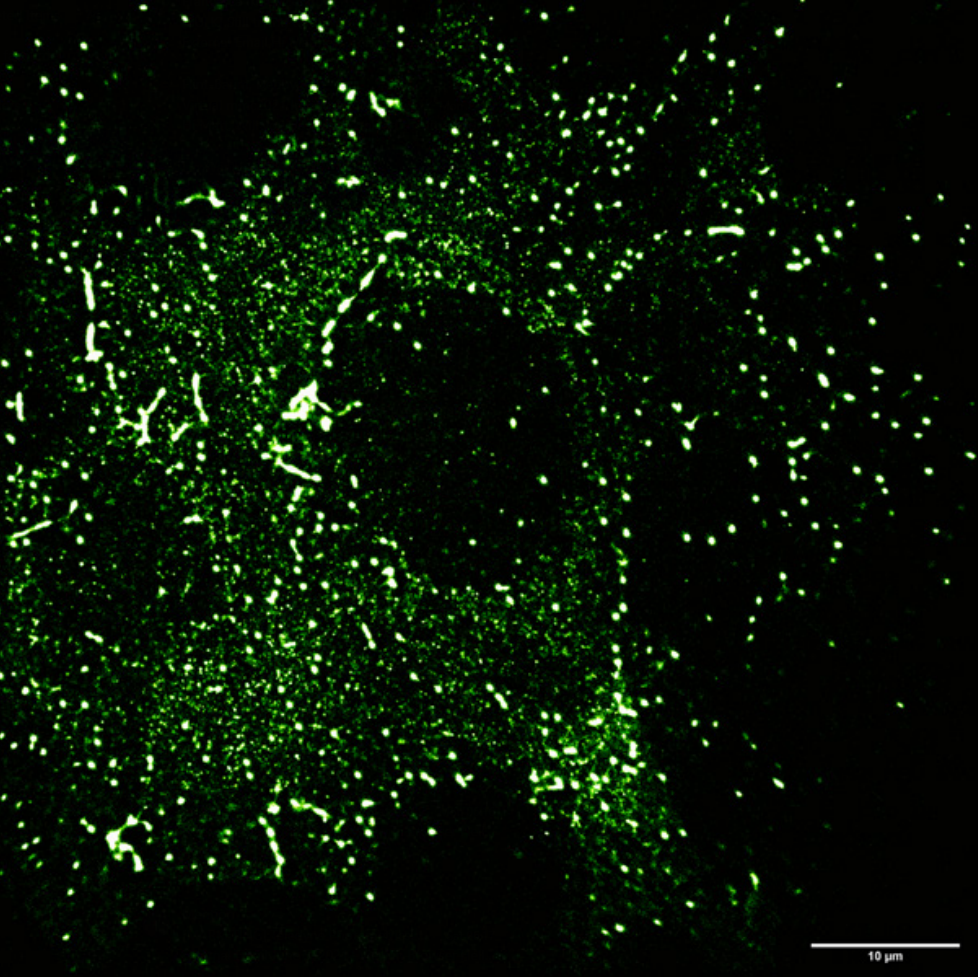
These imaging experiments are focused on proteins that interact and bind to DNA, particularly structural maintenance of chromosome (SMC) proteins. Mr. Barth explains further, “A strand of DNA is tethered to a surface with two biotin handles, in between these handles the DNA is loose and moves around… we flow fluorescently labeled proteins over the DNA, and image protein-DNA and protein-protein interactions with multiple colors.”
The overall project looks at SMC proteins and how they extrude a loop of DNA, with an aim to understand DNA looping and interactions of loop extruders with other DNA-interacting proteins on chromosomes within cells.
Challenge
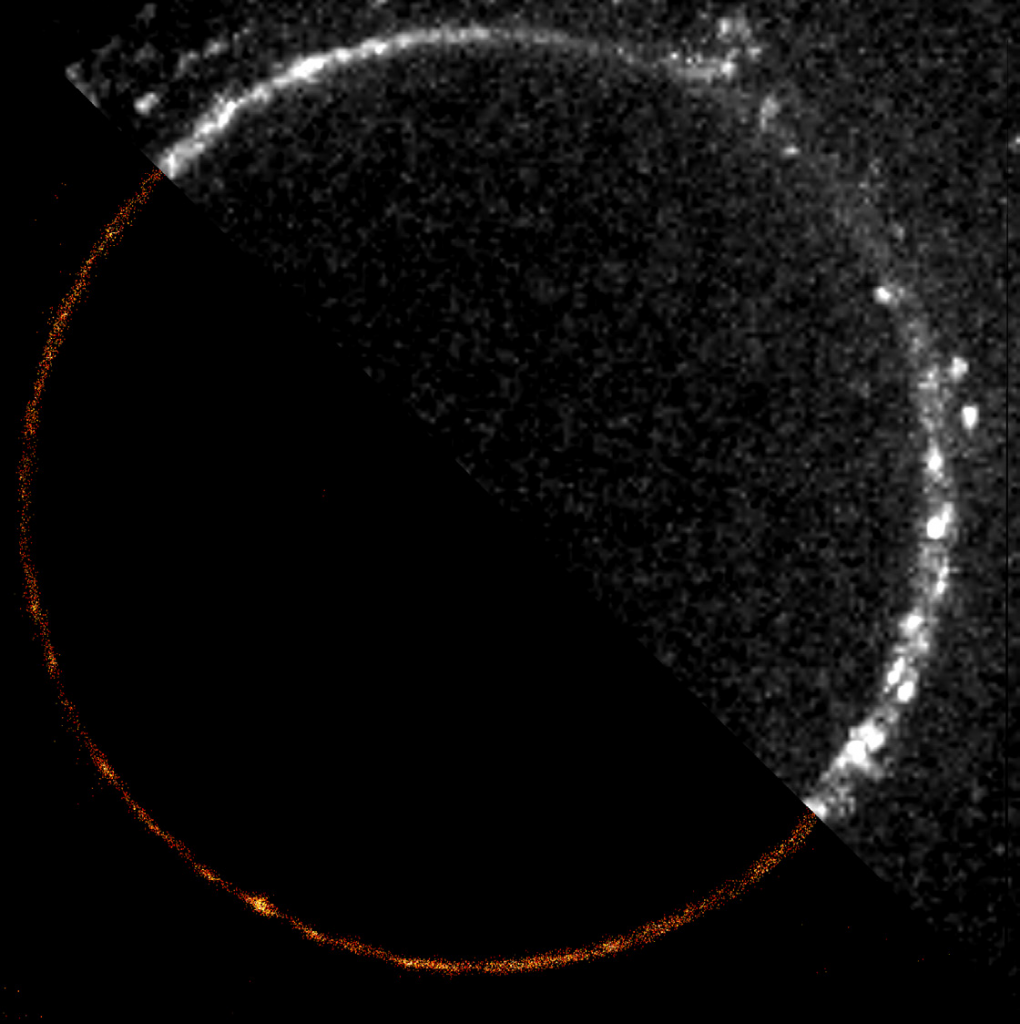
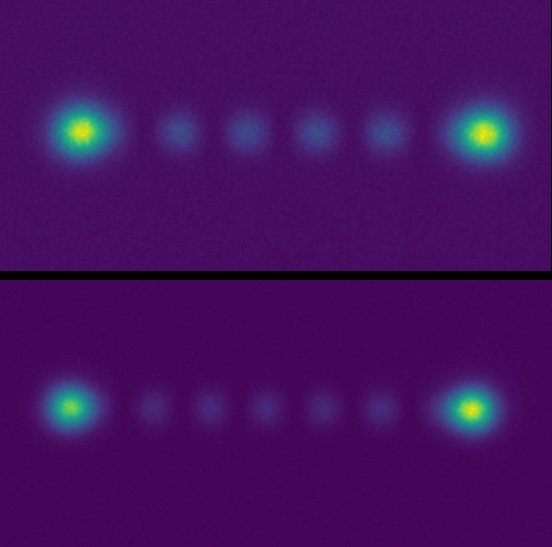
When looking at many DNA strands on a surface, high-throughput imaging is a must. Mr. Barth told us of a previous EMCCD solution that limited throughput, “The field of view was small, just 512×512 pixels, so we just didn’t see much on it. We wanted to enlarge the FOV with a larger camera and more pixels, as well as change our magnification from 100x to 60x as we were over magnifying.”
“The more DNA I can image, the better the statistics per experiment, which is helpful for experiments with a low probability of the event we want to observe. SMC protein loop extrusion usually only happens for a few minutes in vitro, the more events we capture within this critical frame right after protein flush in, the better the statistics and the less often we need to repeat something.”
As the signals from the DNA are bright and sufficient speed can be achieved with 100 ms exposure, the best solution would be a larger field of view (FOV) camera with a small pixel that can maximize throughput while matching to 60x magnification.
We spoke to you immediately and it was quite clear we were going to go with Photometrics… setting up the [Prime BSI Express] was as simple as plugging in a USB.
Solution
The Prime BSI Express is an ideal solution for this application, featuring a 2048×2048 array of small 6.5 μm pixels across a 19 mm diagonal FOV, increasing the available imaging space. Mr Barth spoke about his experiences with the Prime BSI Express CMOS, “60x magnification works well with the [6.5 μm] pixel size of the Prime BSI Express, we are at a pixel size of 100 nm at the sample, just what it should be.”
“We spoke to you immediately and it was quite clear we were going to go with [Teledyne] Photometrics, we didn’t even talk to another company… Setting up [the Prime BSI Express] and letting it talk to the computer was as simple as plugging in a USB, there was almost nothing you can do wrong… totally user-friendly and we see everything we want to see.”
“I was really surprised how small it is, I have a lot of space suddenly! It was also nice that you provided the USB and triggering cables with software, it’s everything you need and works well through MicroManager.”