Dr Maurizio Pezzoli, Prof. Henry Markram
Neural Microcircuitry Laboratory, EPFL, Switzerland
Background
Dr. Maurizio Pezzoli works in the Neural Microcircuitry Laboratory, performing whole-cell patch clamping in acute slices in order to analyze local microcircuitry. The main approach is through multiple patch-clamp, as Dr. Pezzoli says “the lab has the first 12 patch system so we can put 12 pipettes in one slice and see how close neurons talk to each other”.
“The effort of the lab is to try and understand how different types of neurons are connected and how the bigger circuit is connected, my main focus would be around the somatosensory cortex and somatosensory thalamus, but we are also testing some other parts such as hippocampus.”
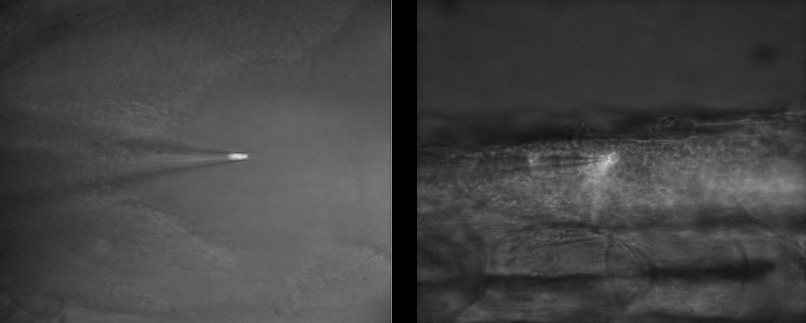
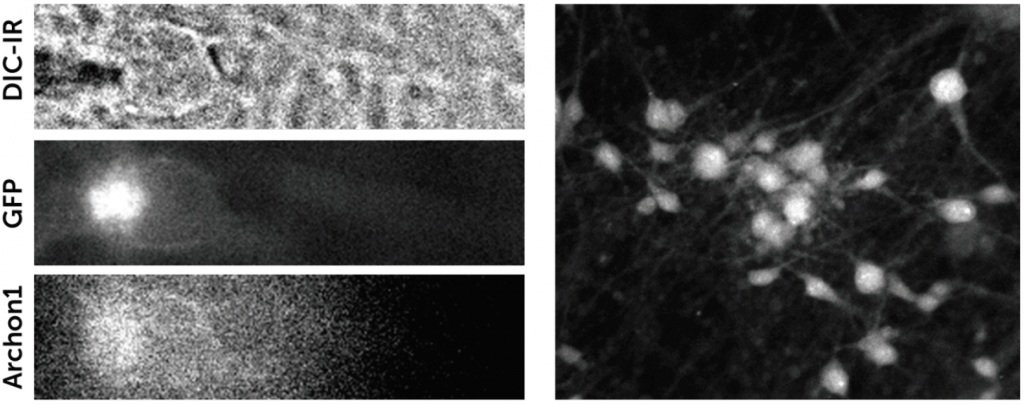
To this end, Dr. Pezzoli performs electrophysiology experiments on ~300 μm slices of brain tissue, using multiple patch pipettes to record neuronal function. However, generating a clear image of the tissue is vitally important to ensure the right neurons are patched with the micropipettes.
Challenge
Dr. Pezzoli outlined some of the challenges of his work: “We need a clear image in an environment that is mostly black and white, we are also working with infrared as this can go through the 300 μm tissue slice… our patching is all done live and you need good optical guidance when approaching with multiple pipettes when cells are in thick tissue, and to see the right time to do the patch, you really need to see things properly.”
“We push the contrast a lot and require a sensitive camera, secondly we need it to be very fast as we only have a small opportunity to patch the cell then it’s gone, we also need great resolution as you’re looking at very tiny surfaces and if you increase the optics too much you lose the working distance, so there are limits. We have a working distance of around 2 mm but as we put more pipettes we are limited in our XY directions.”
This challenging application requires a flexible, highly sensitive camera with a large dynamic range.
Very happy with [the Prime 95B], I was having problems before and this was a great solution, and probably the best optics I have.
Solution
The Prime 95B CMOS is an ideal solution for this application, with a near-perfect 95% QE for maximizing signal collection, and a high speed across a large field of view. The large dynamic range in 16 bit mode will allow for the identification of even the smallest neuron while pushing the brightness and enabling a good contrast in the image, assisting the patch clamping process.
Dr. Pezzoli has been using the Prime 95B for his patch clamping experiments: “It makes the patching easier, the main benefit for me is that it improved my patching as I can see better, particularly with the tiny interneurons…. the setup experience was quite straightforward, it is always nice to receive new equipment in the lab and it worked very easy.”