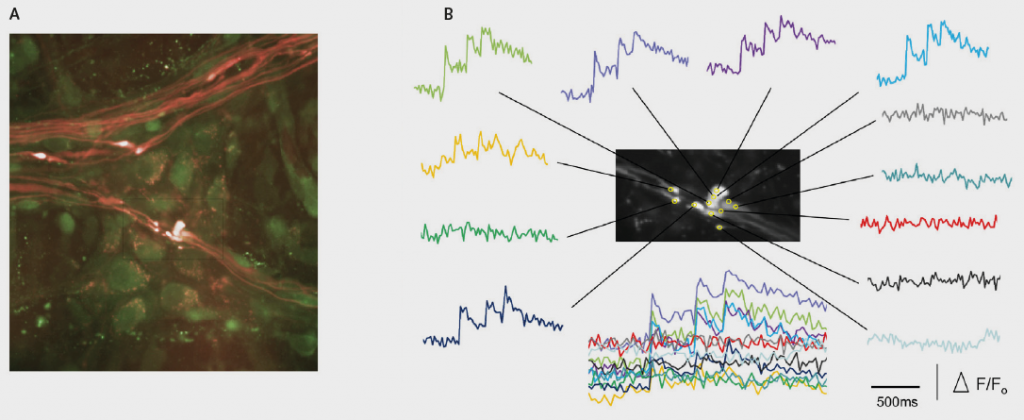
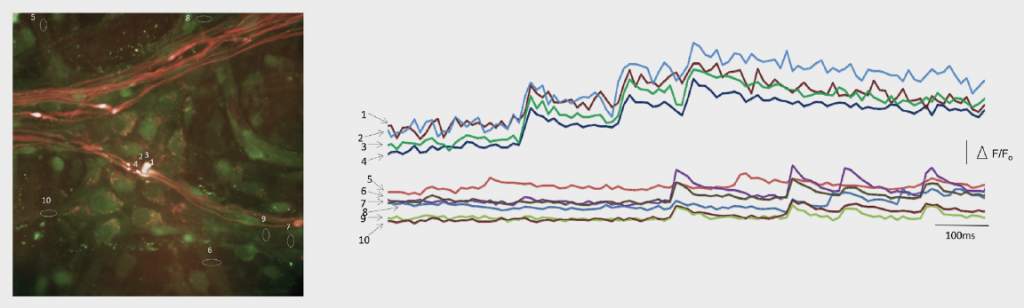
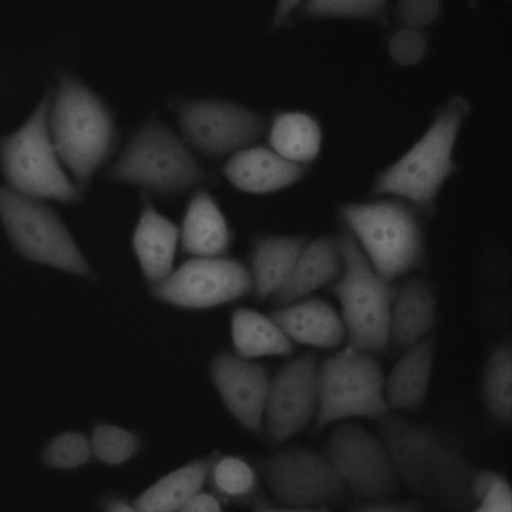
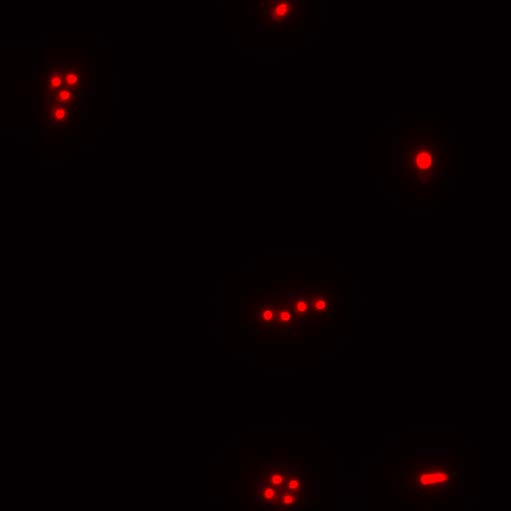
Professor Fei Sun
Institute of Biophysics (IBP), Center for Biological
Imaging (CBI) of Chinese Academy of Sciences (CAS)
Background
The Center for Biological Imaging is a member of Core Facility for Protein Sciences of Chinese Academy of Sciences, which is a support system for scientific research and plays an integral part in supportinginnovation within the Institute of Biophysics. The core facility center has become the largest CAS biological instrument platform in the Beijing area. It is responsible for running and maintaining scientific instruments and providing technical support for the institute’s research.
The center provides services and consultation for more than 100 external research projects, and endeavors to develop improved scientific research methods and innovations in scientific instrumentation. CBI’s research field is primarily related tostructural biology and cell biology. Its goal is to combine various imaging approaches (fluorescence microscopy and cryo-electron microscopy) as well as developing new methodologies to realize the 3D imaging of biological systems from nano-scale to meso-scale in nano-meter resolution.
Challenge
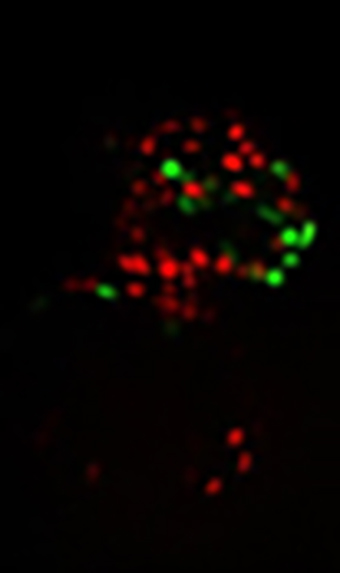
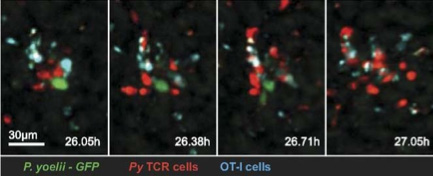
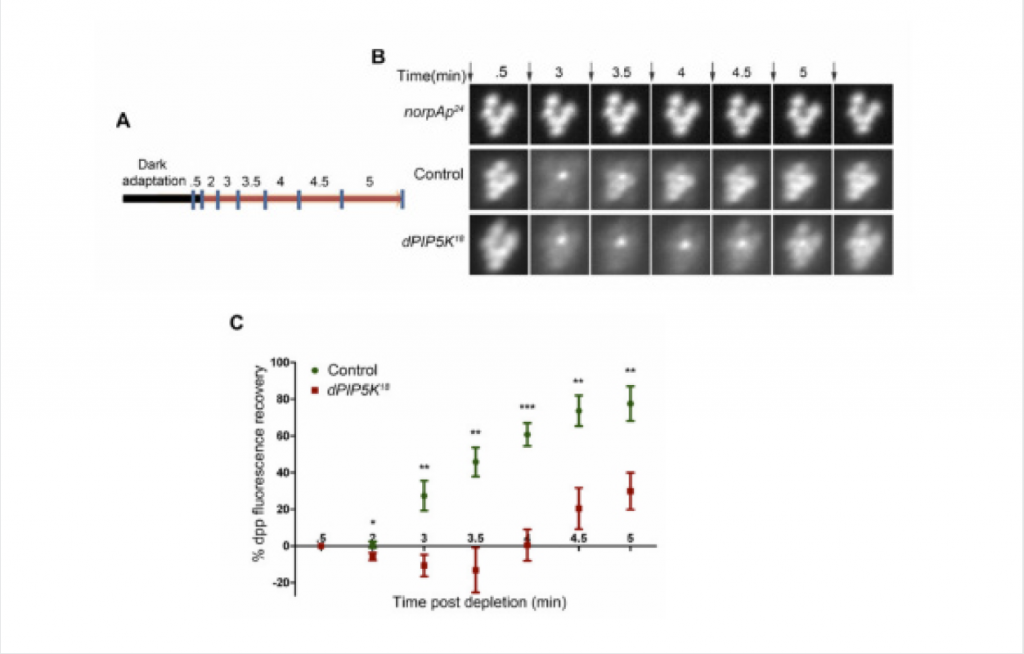
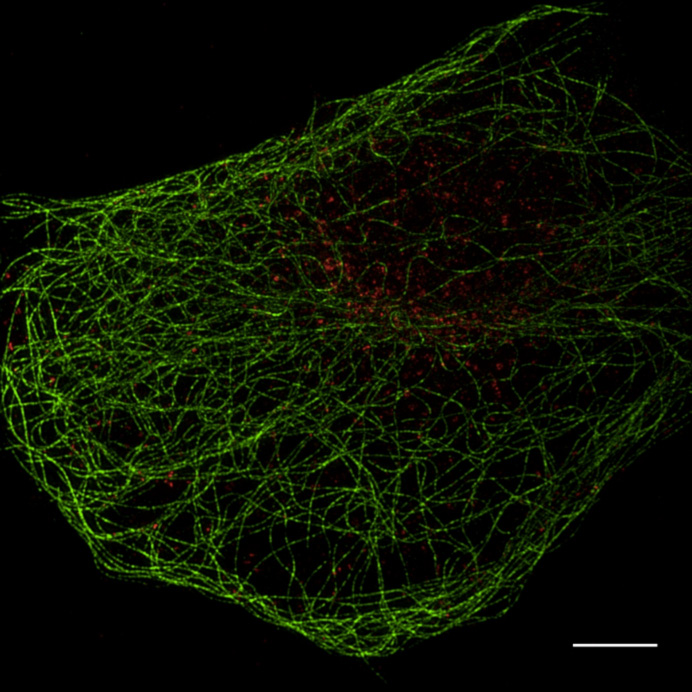
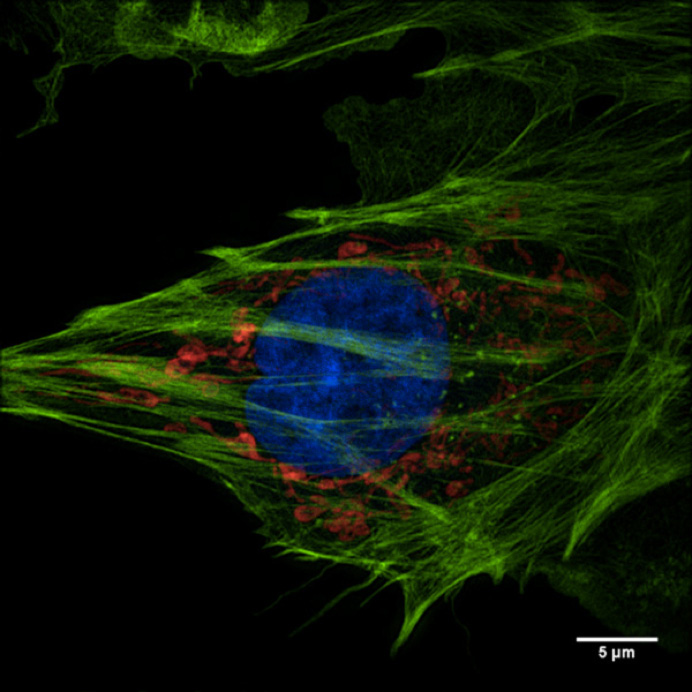
The facility’s research interest is in the advanced technology of optical microscopy and image processing, especially the structured illumination super-resolution microscopy. Their primary focus is on super-resolution light microscopy and biological imaging.
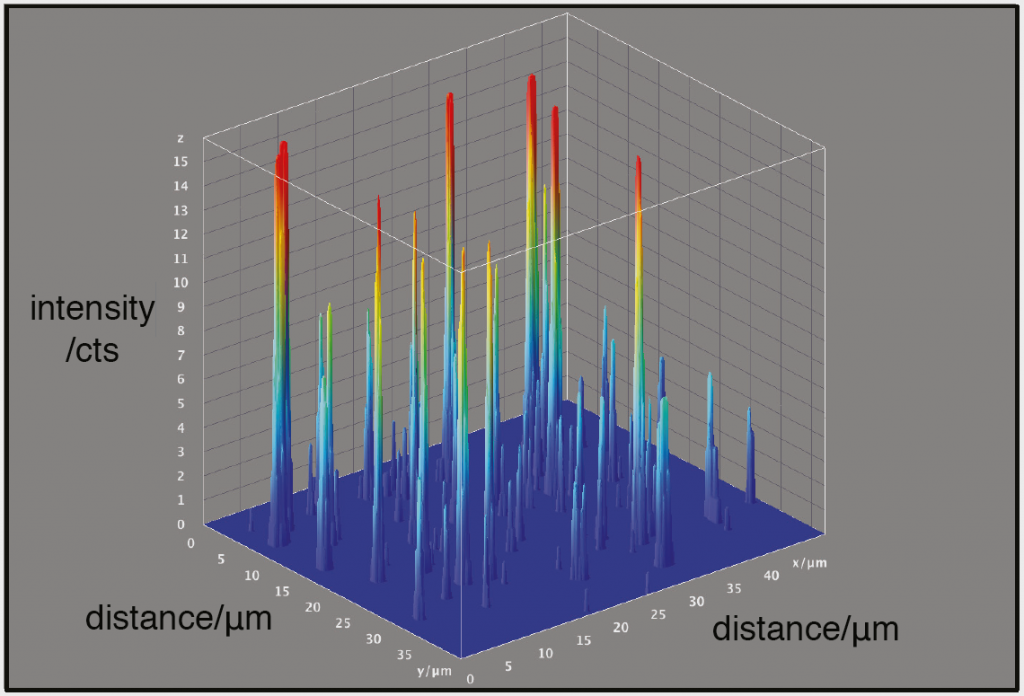
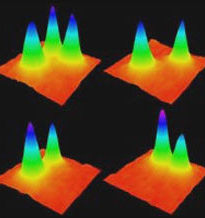
The research team needed to obtain accurate and reproducible data from their imaging system. This would enable them to reconstruct high frequency information from their original images. To accomplish this, their application required a highly-sensitive detector.
To meet and support these stringent imaging requirements, the Evolve® 512 EMCCD camera (new series now available) was recommended by their super resolution system supplier, GE Healthcare.
The Evolve is a very stable camera and provides the high level of accuracy we need for our super resolution microscopy research.
Solution
The Evolve 512 (new series now available) was the first scientific imaging solution that enabled the ability to read out pixel data in photoelectrons. The camera’s ability to output accurate and reproducible data are very favorable for reconstructing higher resolution information.
Because the camera is highly-sensitive, it was very well suited for the facility’s low-light bio-imaging applications too. “The Evolve is a very stable camera and provides the high level of accuracy needed for our super resolution microscopy research,” stated professor Sun.

Further Information
Additional information about the Center for Biological Imaging of Chinese Academy of Sciences is available at: http://cbi.ibp.ac.cn/cbiweb/english/