Introduction
Interferometric scattering (iSCAT) allows for sensitive non-fluorescent detection of nano-scale samples, such as particles as small as 5 nm in diameter. This fills an important niche in biological imaging, allowing the detection of single nano-objects such as viruses, DNA, and proteins. Imaging with iSCAT is label-free, fast, and high resolution, allowing for study of micro-scale samples such as cells, including cell membranes.
The two principles behind iSCAT are interference and scattering, in order for the detection of excitation signals generated by nanoparticles.
Interference
Interference (also known as superposition) is where two or more waves combine. Depending on the phase of these waves, they can combine constructively or destructively (as seen in Fig.1). This interference is temporary and only at the site where the waves meet, as both waves continue to move and will separate from each other, unaffected by the interference.

In the case of microscopy, when samples are illuminated by the light source they will scatter light. This means the total light intensity returning to the microscope is different from the illumination light, as some of the light has been scattered and reflected. With iSCAT, the major variable to measure is the interference of the scattered and reflected light. As microscope samples scatter light and the background does not, light from the background will interfere differently to light from the object. This means the object will be detectable based on the interferometry of the scattered light, which is the principle of iSCAT microscopy.
iSCAT Microscopy
An iSCAT microscope illuminates a sample, which will scatter and reflect the illumination light. This scattered light returns to the microscope, where it interferes with a reference light field at an interface. The interference of a scattered light field with a known reference light field allows for sensitive iSCAT detection of nano-scale samples. This concept is outlined in Fig.2, where the light path in a brightfield microscope is compared to iSCAT.
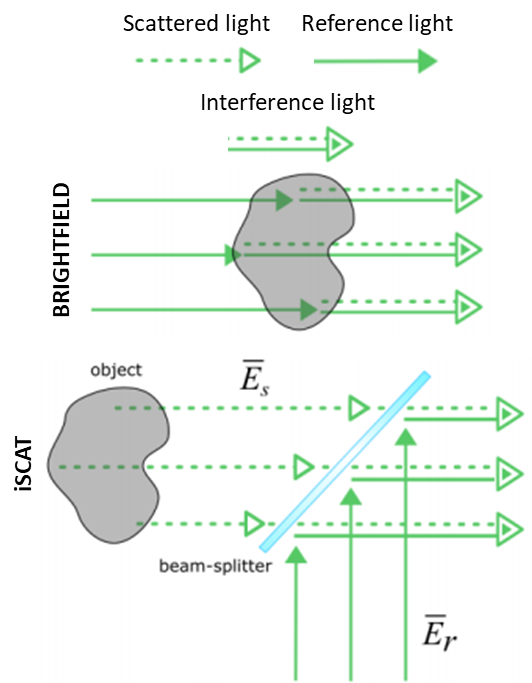
This imaging setup can be achieved easily, by obtaining a few optics, a light source, and a sensitive scientific camera. The reflected light (background) is not rejected, and the signal scales linearly with the scattering amplitude and the sample volume. An example of an iSCAT microscope can be seen in Fig.3.

Applications Of iSCAT
iSCAT microscopy has been successfully used to detect individual unlabelled proteins, but an important consideration to bear in mind when imaging samples of this size is the exponential decrease in signal levels. While a 5 nm nanoparticle is only 10x smaller than a 50 nm nanoparticle, the signal received is 1,000,000x smaller! This makes sensitive detection of ~5 nm particles extremely challenging due to the remarkably low signal and the presence of noise in iSCAT measurements, such as laser intensity noise (due to power fluctuations and beam instabilities) and detector noise (read noise, dark current, photon shot noise). A detector with low levels of noise and high sensitivity is necessary. Fig.5 demonstrates the use of iSCAT when detecting individual unlabeled particles, such as proteins and viruses.

iSCAT also features background removal, in order to pick single proteins or particles out from a large population. iSCAT is extremely sensitive to even the slightest changes in the optical path, down to the level of small proteins. Often the samples imaged with iSCAT microscopes are dynamic, meaning that the background can be removed on a time-scale basis, similar to other super-resolution techniques that use blinking fluorophores in order to localize signals. Background subtraction in order to localize particles with iSCAT can be seen in Fig.4.

iSCAT and TIRF
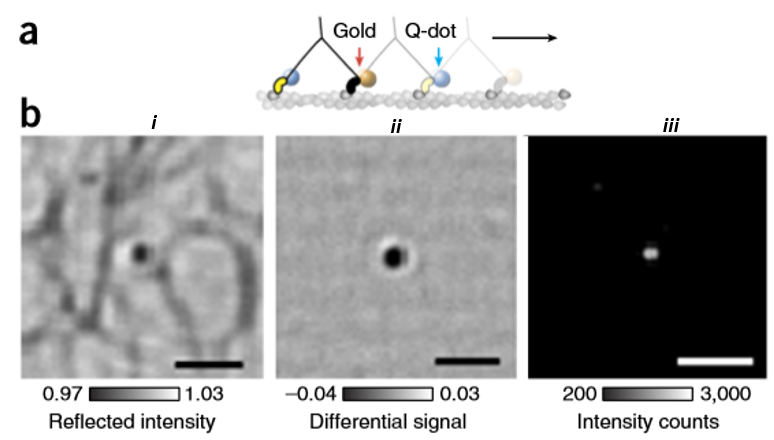
One advantage of iSCAT is its compatibility with the other fluorescence imaging methods such as epifluorescence and total internal reflection fluorescence (TIRF) microscopy. This can be easily done by adding a single dichroic mirror in the light path of the system in which separates the iSCAT wavelength from both fluorescence excitation and emission. By doing so, a single optic can divide the experimental set up into iSCAT and a TIRF microscope which can be used individually or with a combination of each other. An example of the simultaneous iSCAT and TIRF can be seen in Fig.6 where a single myosin 5a molecule has been labeled with a 20 nm gold nanoparticle and a quantum dot respectively. Here, combined TIRF and iSCAT microscopy have been used by taking advantage of the blue fluorescence excitation and red iSCAT illumination. This enables imaging with common fluorophores including Alexa 488, GFP, and quantum dots without suffering perturbations from iSCAT channel.
Tracking Molecules With iSCAT
Apart from the detection of sufficient numbers of photons, one of the most important factors in single-particle tracking is the ability to estimate and remove background from the optical setup and scattering within the sample. For instance, using iSCAT enables tracking of 40 nm GNP labeled membrane proteins with a very small localization precision on thin regions of the axons of neuronal cells. Moreover, taking advantage of the interferometric nature of iSCAT, the 3D motion of the molecules on the tube-like axon structure can be observed, as seen in Fig.7.

Cameras For iSCAT
iSCAT is a powerful method to image nano-scale objects at high resolution which can be combined with other microscopy methods including TIRF. This can be achieved by using a few optics and a sensitive camera. For iSCAT application, high frame rate and high sensitivity cameras which can give a high signal-to-noise ratio can contribute massively to the efficiency of this method. Accordingly, back-illuminated sCMOS cameras will facilitate imaging with very low exposure time and high acquisition speed. The very low noise performance is also important here.
Summary
iSCAT allows for successful, sensitive, and label-free detection of nano-scale samples. iSCAT features localization with outstanding spatial and temporal resolution, made possible by a high signal-to-noise ratio (SNR). By using the light scattered from the sample, iSCAT bypasses the need for fluorescence, instead using elegant physics to generate a powerful technique that can see incredibly small samples at super-resolution levels.
References
Gemeinhardt, A., McDonald, M. P., König, K., Aigner, M., Mackensen, A., & Sandoghdar, V. (2018). Label-Free Imaging of Single Proteins Secreted from Living Cells via iSCAT Microscopy. Journal of Visualized Experiments, (141), 1–10.
Ortega Arroyo, J., Cole, D., & Kukura, P. (2016). Interferometric scattering microscopy and its combination with single-molecule fluorescence imaging. Nature Protocols, 11(4), 617–633.
Ortega-Arroyo, J., & Kukura, P. (2012). Interferometric scattering microscopy (iSCAT): New frontiers in ultrafast and ultrasensitive optical microscopy. Physical Chemistry Chemical Physics, 14(45), 15625–15636.
Piliarik, M., & Sandoghdar, V. (2014). Direct optical sensing of single unlabelled proteins and super-resolution imaging of their binding sites. Nature Communications, 5, 1–8
Taylor, R. W., & Sandoghdar, V. (2018). Interferometric Scattering (iSCAT) Microscopy & Related Techniques. 1–42.
Young, G., & Kukura, P. (2019). Interferometric Scattering Microscopy. Annual Review of Physical Chemistry, 70(1), 331–352.
